1. Introduction
In food and pharma industries hygiene is a must, non compliance can
lead to non quality, recalls or even legal pursuits. Hygienic design
must be seen comprehensively for the whole factory, including
building design, air handling, zoning, equipment design... One of
the basics is to ensure that the piping that will be conveying
the product is hygienically designed.
Hygienic piping design will indeed help in the following :
- Avoid materials accumulation that can spoil over time and
contaminate the finished product
- Optimize changeovers efficiency and duration
- Allow more efficient and shorter cleaning time, or even allow
the implementation of a CIP (Cleaning In Place) system
- Prevent foreign bodies generation from pipe parts
This page is focusing on good practices to apply when designing a
hygienic piping system.
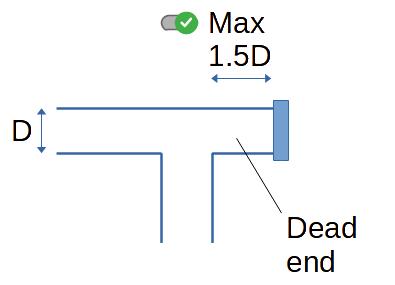
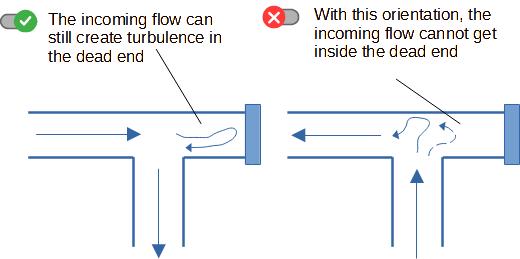
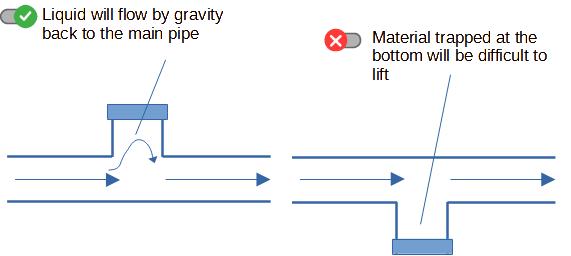
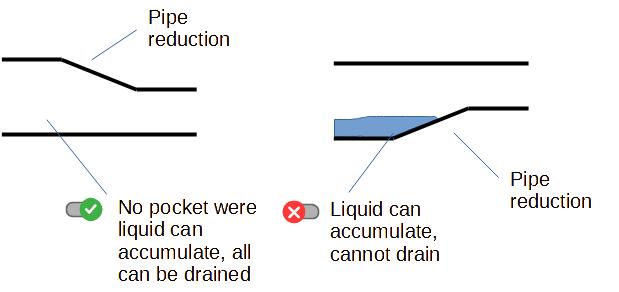
2. Avoiding dead ends
That is a fundamental of hygienic pipe design : avoid areas
where there will be no flow (those areas are also called "dead
ends").
If there is no flow, product will accumulate, deposit, have a long
residence time and will be prone to spoilage. It means also that
those parts of the pipes cannot be reached by cleaning agents for
example during CIP (Cleaning In Place), or can even be difficult to
access for operators manually cleaning the pipes in COP (Cleaning
Out of Place).
3. Pipe roughness and welding quality
Having a smooth surface will reduce the risk to harbor dirt and
bacteria in the asperities of the pipe. It is why a pipe with a
surface roughness < Ra = 0.8 microns will be recommended. The
material must be Stainless Steel to avoid risks of rusting ; the
correct grade of Stainless Steel must be chosen, especially if
the pipe will have to handle corrosive materials during cleaning
sequences for instance.
It is necessary to weld couplings to the pipe in order to be able
to assemble it. Welding needs to be controlled to make sure they
will not release foreign bodies, or harbor micro organisms due to a
rough surface. Welding must be :
- Done under inert gas coverage
- Smooth / polished
- Continuous (no gaps, no crevices)
The use of orbital cutting and welding is recommended over a fully
manual operation that will be less precise. It is recommended to
check individually all the pipes welding before
installation.
4. Pipe coupling
Designing and assembling properly pipe couplings is very important
to implement an hygienic pipe that will be easy to clean.
Coupling should be chosen and design to present the following
characteristics :
- Avoid gaps where product could accumulate
- Be designed to avoid overcompression of the gaskets, this could
damage the gasket, or push it on the inside of the pipe where it
could generate foreign bodies
- Ease the alignment of the 2 pipe ends when assembling, will
prevent gaps or on the contrary parts protruding on the inside of
the pipe
- Preferably be easily dismantable to allow inspection and
cleaning.
In practice, on the line, the operators must be careful to avoid
pipe misalignment (if the coupling are not designed to avoid it).
Especially having a gasket protruding on the inside of the pipe can
generate foreign body if the gasket starts to be damaged, foreign
bodies are a major cause of recall of products for pharma and food
industries.
5. Valves
Simple valves design must be preferred when on the product flow in
hygienic piping, typically butterfly valves.
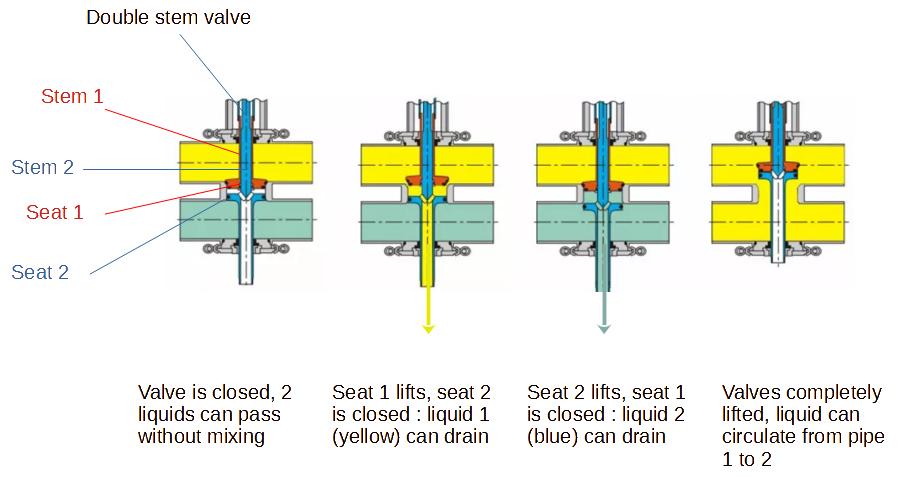
It is also possible to use a new type of valves, called mixproof
valves, which have also the advantage to be able to route safely
different products at the same time or solutions used for CIP ;
those valves are also designed to automatically clean the seats of
the valve during CIP cycle. Mixproof valves have a leak detection
feature, when one of the seats starts to leak, the product will be
drained, which will be visible.
6. Design Calculations for Hygienic Piping
6.1 Pressure Drop (ΔP)
The total pressure loss along a pipe run is the sum of frictional
loss and minor losses (valves, bends, reducers). Use the
Darcy‑Weisbach equation:
ΔP_friction = f·(L/D)·(ρ·V²/2)
- f – Darcy friction factor (Moody chart or
Colebrook‑White equation).
- L – Length of straight pipe (m).
- D – Nominal pipe diameter (m).
- ρ – Fluid density (kg·m⁻³).
- V – Mean velocity (m·s⁻¹).
6.2 Reynolds Number (Re) & Flow Regime
Re determines whether the flow is laminar (Re < 2000)
or turbulent (> 4000). For hygienic systems a turbulent regime
(Re ≈ 10 000–30 000) is preferred because it enhances mixing and
CIP effectiveness.
Re = (ρ·V·D)/μ
- μ – Dynamic viscosity (Pa·s).
6.3 Velocity Recommendation
Target a superficial velocity of 1–1.5 m s⁻¹.
This range balances shear stress (preventing product hold‑up) and
energy consumption.
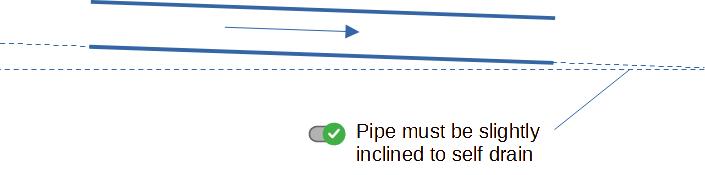
6.4 Minimum Self‑Drain Length
To guarantee complete drainage of dead‑end sections, keep the
length L_dead ≤ 1.5 × D (as already noted). For inclined
runs, a slope of 1–2 % ensures gravity‑assisted drainage.
7. Standards & Regulatory Guidance
Searches
for “hygienic piping standards” consistently surface the following
documents:
- 3‑A Sanitary Standards – Covers pipe
material, surface finish (Ra ≤ 0.8 µm), and joint design.
- ASME BPE (Bioprocessing Equipment) – Provides
detailed guidance on CIP/SIP, valve selection, and allowable L/D
ratios (typically ≤ 4:1).
- ISO 14644‑1 – Cleanroom classification,
influencing piping layout and accessibility.
- FDA 21 CFR 211 – Good Manufacturing Practice
(GMP) requirements for pharmaceutical equipment.
8. CFD & Simulation for Optimisation
If the hygiene of the piping is extremely critical, some
simulations can be done on Computational Fluid Dynamics (CFD) to
verify that:
- No stagnant zones exist (visualised with residence‑time
contours).
- Shear rates exceed the threshold needed for biofilm removal
during CIP (≥ 500 s⁻¹ for most dairy products).
- Pressure drop predictions match hand calculations within
± 10 %.
Typical workflow:
- Create a 3‑D CAD model of the pipe network (including
reducers, elbows, and valves).
- Apply a turbulence model (k‑ε or SST) and set wall roughness
to 0.8 µm.
- Run a steady‑state simulation for normal operation, then a
transient CIP simulation with hot water (80 °C) and caustic
solution.
9. Maintenance, Validation & Documentation
9.1 Routine Inspection Checklist
- Verify surface roughness (Ra ≤ 0.8 µm) using a profilometer.
- Inspect weld seams for porosity or cracks (radiographic or
ultrasonic testing).
- Confirm that all instrumentation ports are fully wetted during
CIP (use dye‑trace tests).
- Record torque values on couplings to avoid over‑compression of
gaskets.
9.2 Validation Protocols
For GMP environments, a Installation Qualification (IQ),
Operational Qualification (OQ), and Performance
Qualification (PQ) are mandatory. Include the following
acceptance criteria:
- Maximum residual product after CIP < 0.1 mg L⁻¹.
- Pressure drop variation < 5 % between OQ runs.
- No detectable biofilm after 30 days of operation (ATP assay).
10. Frequently Asked Questions (FAQ)
- What is the ideal pipe roughness for hygienic systems?
- Ra ≤ 0.8 µm (per 3‑A and ASME BPE). Anything higher increases
microbial adhesion.
- How long can a dead‑end segment be before it becomes a
contamination risk?
- Keep dead‑end length ≤ 1.5 × diameter. For larger diameters,
consider installing a dedicated purge line.
- Which valve type offers the best CIP performance?
- Mix‑proof (dual‑seated) or hygienic butterfly valves with
automated seat‑cleaning cycles are preferred.
- Do I need to calculate Reynolds number for every branch?
- Yes, especially for branches feeding low‑viscosity liquids;
turbulent flow (Re > 10 000) improves cleaning efficiency.
- Is orbital welding mandatory?
- While not legally required, orbital welding provides
repeatable, low‑roughness joints that meet GMP expectations.